OCS
Introduction.
Lymphocytic hypereosinophilic syndrome (L-HES) is an indolent T-cell lymphoproliferative disorder characterized by chronic blood hypereosinophilia and eosinophil-related organ damage secondary to the production of eosinophilopoietic cytokines (including interleukin-5) by clonal T cells, among which CD3-CD4+ T cells are the most frequent subset. Both high exposure to oral corticosteroids (OCS), failure and/or toxicity of OCS-sparing therapies and the risk of progression to AITL (up to 5 - 10% of patients) underscore the unmet need for novel treatments targeting the clonal T-cells (and not only eosinophils). We previously reported that peripheral CD3-CD4+ T cells express C-C chemokine receptor 4 (CCR4). We hypothesized that mogamulizumab, an afucosylated monoclonal antibody targeting CCR4-positive cells through antibody-dependent cellular cytotoxicity, could be beneficial for refractory L-HES patients.
Methods.
We assessed both the expression of CCR4 by clonal CD3-CD4+ T cells in the skin and lymph nodes as well as theability of mogamulizumab to induce ADCC in vitro on sorted CD3-CD4+ T cells from two L-HES patients, using homologous sorted NK cells as effector cells. We then set up a compassionate use program of intravenous mogamulizumab (1.0 mg/kg weekly for the first 28-day cycle, then on days 1 and 15 from subsequent cycles) for refractory L-HES patients despite treatment with immunosuppressants and/or biologics targeting the IL-5 pathway. Complete blood response was defined as complete if the subset of CD3-CD4+ T cells was <0.5% of all lymphocytes, or as partial if the number of CD3-CD4+ T cells decreased by >50% without reaching complete response at the time of the last perfusion.
Results.
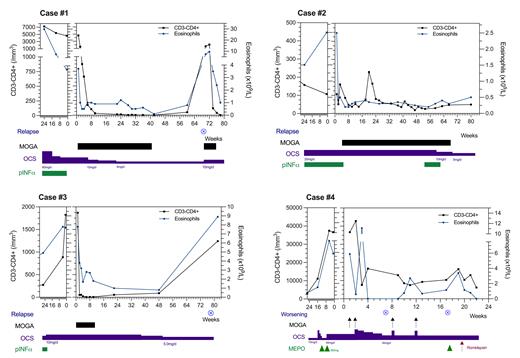
Significant CCR4 expression on tissue CD4+ T cells was evidenced, and NK cells indeed led to the lysis of CD3-CD4+ T-cells coated with mogamulizumab (10 μg/ml). Four patients with refractory L-HES (despite treatment with high-dose OCS, n=4; pegylated interferon alpha 2a; n=3; or mepolizumab, a single patient) and active disease entered the compassionate use program (Table 1). Despite substantial tapering in OCS doses, all cases showed clinical response. Blood responses (either partial or complete, two patients each) and sharp decrease in absolute eosinophil counts (AEC) were reported in all patients ( Figure 1). Both patients with lymph node enlargement rapidly improved. Although prolonged blood and clinical responses have been observed after discontinuation of mogamulizumab, relapses still occurred preceded by an increase in T-cell clone size and absolute eosinophil count (AEC). Rechallenging a relapse with a short course of mogamulizumab resulted in a complete remission for a further 12 months in case #1. In terms of safety, mogamulizumab was discontinued in two patients due to adverse events: one for recurrent infusion-related reactions and one for infectious events. Lymphopenia was reported in 3/4 cases but was preexisting in the 3 cases. Case #4 reported a serious adverse event related to a tumor lysis syndrome leading to the massive release of IL-5 and subsequent rebound of AEC. Hence lower doses of mogamulizumab, transient use of OCS and/or mepolizumab should be considered in patients with very high number of CD3-CD4+ T cells who are about to start mogamulizumab.
Discussion.
Mogamulizumab represents a new therapeutic option for L-HES patients with severe refractory HES, T-cell clone related symptoms and/or large or rapidly increasing clonal T-cell counts. In patients who achieve complete remission under mogamulizumab, whether fixed repeated (e.g., every 6 months) infusions could be beneficial to prevent relapses and transformation into high-grade lymphoma remains to be investigated.
Figure legend. Hematological responses to mogamulizumab in four refractory lymphocytic HES patients.
oral corticosteroids; pINF-α: pegylated interferon alpha; MEPO: mepolizumab; MOGA: mogamulizumab.
OffLabel Disclosure:
Groh:AstraZeneca: Consultancy; GlaxoSmithKline: Consultancy, Honoraria. Terriou:Alexion, AstraZeneca Rare Disease: Consultancy, Membership on an entity's Board of Directors or advisory committees. Kahn:AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline: Consultancy, Membership on an entity's Board of Directors or advisory committees. Lefèvre:AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; GlaxoSmithKline: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Mogamulizumab for CD3-CD4+ lymphocytic Hypereosinophilic Syndrome.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal